Green hydrogen Production from the oxidative reform of clean biogas over Ni/MgO-Nb2O5 catalysts
DOI:
https://doi.org/10.34024/jsse.2023.v1.15904Keywords:
methane, biogas, synthesis gas, catalysts, nickel, niobiumAbstract
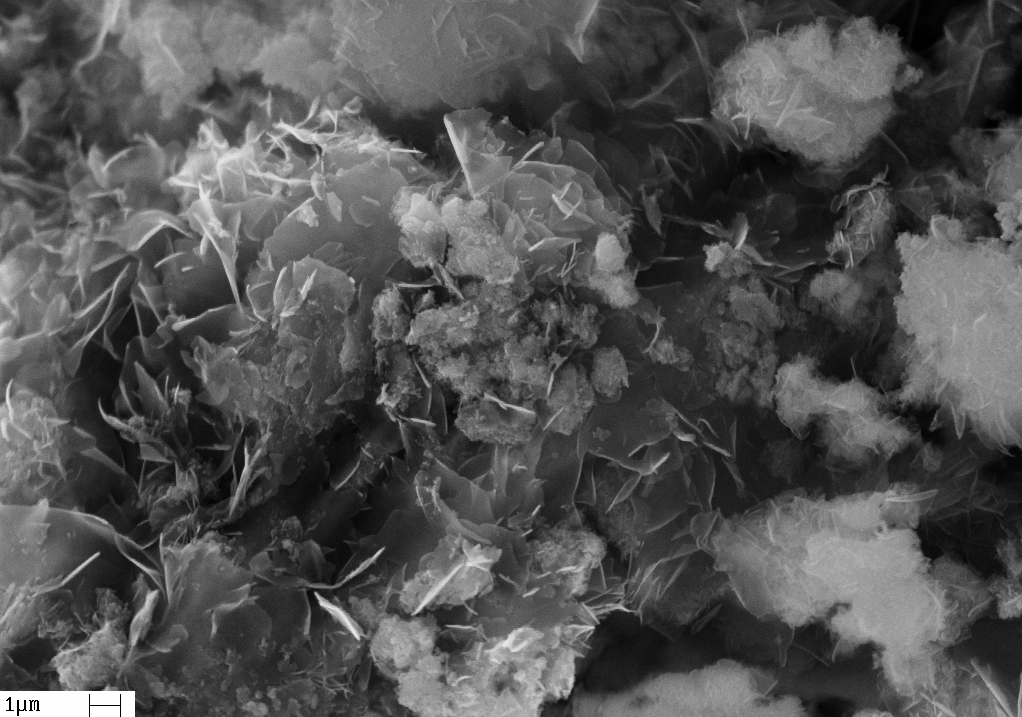
Synthesis gas has a variety of applications ranging from its transformation into Fischer-Tropsch fuels, its processing to produce pure hydrogen, and even its direct combustion to generate energy, among many other applications. The objective of this work was the conversion of a model of biogas (clean biogas) into synthesis gas, H2 /CO, through oxidative reforming of methane over NiO/MgO/Nb2O5 catalysts. The catalysts in this work were prepared by impregnation and calcination (at 750ºC) and subsequently characterized by Energy Dispersive X-ray spectroscopy (EDX), Scanning Electron Microscopy (SEM), X-ray diffraction (XRD), Nitrogen Adsorption-Desorption (BET method) and Temperature Programmed Reduction with H2 (TPR). Finally, these catalysts were tested in the oxidative reform of methane (molar ratio of 1.5CH4:1CO2:0.25O2) at 750 ºC, 100mg of catalyst at a total flow of 110 mL.min-1, and 1 atm (inside the reactor). According to the results, it was verified that the catalyst composed of the NiO/MgO mixture is composed of the NiO-MgO solid solution, whereas the NiO/MgO/Nb2O5 catalysts are also formed by nickel niobate (NiNb2O6). All catalysts showed catalytic activity in the oxidative form of biogas, NiMg, Ni60NbMg, and Ni40NbMg catalysts showed the highest conversion value. The efficiency of the catalysts in the oxidative reforming of biogas improved gradually as the %MgO increased in the NiO/MgO/Nb2O5 system. The characterization of the catalyst after the reaction demonstrated that the carbon formed is of the filamentous type.
References
E. Winquist, P. Rikkonen, J. Pyysiäinen, and V. Varho, “Is biogas an energy or a sustainability product? - Business opportunities in the Finnish biogas branch,” J Clean Prod, vol. 233, 2019, doi: 10.1016/j.jclepro.2019.06.181.
Y. J. O. Asencios, C. B. Rodella, and E. M. Assaf, “Biomethane reforming over Ni catalysts supported on PrO2-ZrO2 solid-solutions,” Journal of CO2 Utilization, vol. 61, p. 102018, Jul. 2022, doi: 10.1016/j.jcou.2022.102018.
I. A. Cruz et al., “Valorization of cassava residues for biogas production in Brazil based on the circular economy: An updated and comprehensive review,” Cleaner Engineering and Technology, vol. 4. 2021. doi: 10.1016/j.clet.2021.100196.
Y. J. O. Asencios, K. F. M. Elias, and E. M. Assaf, “Oxidative-reforming of model biogas over NiO/Al 2 O 3 catalysts: The influence of the variation of support synthesis conditions,” Appl Surf Sci, vol. 317, 2014, doi: 10.1016/j.apsusc.2014.08.058.
Y. J. O. Asencios et al., “Partial Oxidation of Bio-methane over Nickel Supported on MgO–ZrO2 Solid Solutions,” Top Catal, 2023, doi: 10.1007/s11244-023-01822-7.
A. L. D. Ramos, J. J. Marques, V. Dos Santos, L. D. S. Freitas, R. G. V. De Melo Santos, and M. De Mattos Vieira Mello Souza, “Atual estágio de desenvolvimento da tecnologia gtl e perspectivas para o Brasil,” Quim Nova, vol. 34, no. 10, 2011, doi: 10.1590/s0100-40422011001000004.
Y. J. O. Asencios, J. D. A. Bellido, and E. M. Assaf, “Synthesis of NiO-MgO-ZrO2 catalysts and their performance in reforming of model biogas,” Appl Catal A Gen, vol. 397, no. 1–2, 2011, doi: 10.1016/j.apcata.2011.02.023.
Y. J. O. Asencios and E. M. Assaf, “Combination of dry reforming and partial oxidation of methane on NiO-MgO-ZrO2 catalyst: Effect of nickel content,” Fuel Processing Technology, vol. 106, 2013, doi: 10.1016/j.fuproc.2012.08.004.
Y. J. O. Asencios and E. M. Assaf, “Combination of dry reforming and partial oxidation of methane on NiO-MgO-ZrO2 catalyst: Effect of nickel content,” Fuel Processing Technology, vol. 106, 2013, doi: 10.1016/j.fuproc.2012.08.004.
Y. J. O. Asencios, P. A. P. Nascente, and E. M. Assaf, “Partial oxidation of methane on NiO-MgO-ZrO 2 catalysts,” Fuel, vol. 97, 2012, doi: 10.1016/j.fuel.2012.02.067.
G. Y. Boyarko, “Dynamics of global production and commodity flows of niobium raw materials,” Bulletin of the Tomsk Polytechnic University, Geo Assets Engineering, vol. 330, no. 10, 2019, doi: 10.18799/24131830/2019/10/2318.
V. R. Choudhary and A. S. Mamman, “Energy efficient conversion of methane to syngas over NiO-MgO solid solution,” Appl Energy, vol. 66, no. 2, 2000, doi: 10.1016/S0306-2619(99)00039-2.
E. Ruckenstein, “Binary MgO-based solid solution catalysts for methane conversion to syngas,” Catal Rev Sci Eng, vol. 44, no. 3, 2002, doi: 10.1081/CR-120005742.
Y. H. Hu and E. Ruckenstein, “The characterization of a highly effective NiO / MgO solid solution catalyst in the CO2 reforming of CH4,” Catal Letters, vol. 43, no. 1–2, 1997, doi: 10.1023/a:1018982304573.
R. D. Shannon, “Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides,” Acta Crystallographica Section A, vol. 32, no. 5, 1976, doi: 10.1107/S0567739476001551.
K. Kunimori, H. Oyanagi, and H. Shindo, “Nickel-niobia interaction induced by the reduction of NiNb2O6 supported on SiO2,” Catal Letters, vol. 21, no. 3–4, 1993, doi: 10.1007/BF00769480.
L. Guochang, L. Peraldo Bicelli, G. Razzini, and R. Borromei, “Surface investigation of NiNb2O6 electrodes for water photoelectrolysis,” Mater Chem Phys, vol. 23, no. 5, 1989, doi: 10.1016/0254-0584(89)90091-6.
A. Martínez-De La Cruz, N. L. Alcaraz, A. F. Fuentes, and L. M. Torres-Martínez, “Electrochemical lithium insertion in some niobates MNb2O6 (M = Mn, Co, Ni, Cu, Zn and Cd),” J Power Sources, vol. 81–82, 1999, doi: 10.1016/S0378-7753(99)00198-6.
K. Li and D. Xue, “Estimation of electronegativity values of elements in different valence states,” Journal of Physical Chemistry A, vol. 110, no. 39, 2006, doi: 10.1021/jp062886k.
A. C. Faro, P. Grange, and A. C. B. dos Santos, “Niobia-supported nickel-molybdenum catalysts: Characterisation of the oxide form,” Physical Chemistry Chemical Physics, vol. 4, no. 16, 2002, doi: 10.1039/b202517e.
T. V. Choudhary and V. R. Choudhary, “Energy-efficient syngas production through catalytic oxy-methane reforming reactions,” Angewandte Chemie - International Edition, vol. 47, no. 10. 2008. doi: 10.1002/anie.200701237.
V. R. Choudhary, K. C. Mondal, and T. V. Choudhary, “Oxy-CO2 reforming of methane to syngas over CoOx/MgO/SA-5205 catalyst,” Fuel, vol. 85, no. 17–18, 2006, doi: 10.1016/j.fuel.2006.04.013.
V. R. Choudhary and A. S. Mamman, “Oxidative conversion of methane to syngas over NiO/MgO solid solution supported on low surface area catalyst carrier,” Fuel Processing Technology, vol. 60, no. 3, 1999, doi: 10.1016/S0378-3820(99)00046-6.
V. R. Choudhary, B. S. Uphade, and A. S. Mamman, “Simultaneous steam and CO2 reforming of methane to syngas over NiO/MgO/SA-5205 in presence and absence of oxygen,” Appl Catal A Gen, vol. 168, no. 1, 1998, doi: 10.1016/S0926-860X(97)00331-1.
V. C. H. Kroll, H. M. Swaan, S. Lacombe, and C. Mirodatos, “Methane reforming reaction with carbon dioxide over Ni/SiO2 catalyst: II. A mechanistic study,” J Catal, vol. 164, no. 2, 1996, doi: 10.1006/jcat.1996.0395.
J. D. A. Bellido and E. M. Assaf, “Effect of the Y2O3-ZrO2 support composition on nickel catalyst evaluated in dry reforming of methane,” Appl Catal A Gen, vol. 352, no. 1–2, 2009, doi: 10.1016/j.apcata.2008.10.002.
J. D. A. Bellido, J. E. De Souza, J. C. M’Peko, and E. M. Assaf, “Effect of adding CaO to ZrO2 support on nickel catalyst activity in dry reforming of methane,” Appl Catal A Gen, vol. 358, no. 2, 2009, doi: 10.1016/j.apcata.2009.02.014.
E. Ruckenstein and Y. H. Hu, “Carbon dioxide reforming of methane over nickel/alkaline earth metal oxide catalysts,” Appl Catal A Gen, vol. 133, no. 1, 1995, doi: 10.1016/0926-860X(95)00201-4.
E. Ruckenstein and Y. H. Hu, “Methane partial oxidation over NiO/MgO solid solution catalysts,” Appl Catal A Gen, vol. 183, no. 1, 1999, doi: 10.1016/S0926-860X(99)00047-2.
Y. H. Hu and E. Ruckenstein, “An optimum NiO content in the CO2 reforming of CH4 with NiO/MgO solid solution catalysts,” Catal Letters, vol. 36, no. 3–4, 1996, doi: 10.1007/BF00807611.
Y. H. Hu and E. Ruckenstein, “Catalyst temperature oscillations during partial oxidation of methane,” Ind Eng Chem Res, vol. 37, no. 6, 1998, doi: 10.1021/ie980027f.
C. E. M. Guarido, D. V. Cesar, M. M. V. M. Souza, and M. Schmal, “Ethanol reforming and partial oxidation with Cu/Nb2O5 catalyst,” Catal Today, vol. 142, no. 3–4, 2009, doi: 10.1016/j.cattod.2008.08.030.
R. Wojcieszak, A. Jasik, S. Monteverdi, M. Ziolek, and M. M. Bettahar, “Nickel niobia interaction in non-classical Ni/Nb2O5 catalysts,” J Mol Catal A Chem, vol. 256, no. 1–2, 2006, doi: 10.1016/j.molcata.2006.04.053.
A. Guerrero-Ruiz, A. Sepúlveda-Escribano, and I. Rodríguez-Ramos, “Cooperative action of cobalt and MgO for the catalysed reforming of CH4 with CO2,” Catal Today, vol. 21, no. 2–3, 1994, doi: 10.1016/0920-5861(94)80178-9.
J. H. Bitter, K. Seshan, and J. A. Lercher, “The state of zirconia supported platinum catalysts for CO2/CH4 reforming,” J Catal, vol. 171, no. 1, 1997, doi: 10.1006/jcat.1997.1792.
J. H. Bitter, K. Seshan, and J. A. Lercher, “Mono and bifunctional pathways of CO2/CH4 reforming over Pt and Rh based catalysts,” J Catal, vol. 176, no. 1, 1998, doi: 10.1006/jcat.1998.2022.
J. M. Wei, B. Q. Xu, J. L. Li, Z. X. Cheng, and Q. M. Zhu, “Highly active and stable Ni/ZrO2 catalyst for syngas production by CO2 reforming of methane,” Appl Catal A Gen, vol. 196, no. 2, 2000, doi: 10.1016/S0926-860X(99)00504-9.
Z. Hou, O. Yokota, T. Tanaka, and T. Yashima, “A novel KCaNi/α-Al2O3 catalyst for CH 4 reforming with CO2,” Catal Letters, vol. 87, no. 1–2, 2003, doi: 10.1023/A:1022849009431.
V. V. Chesnokov, V. I. Zaikovskii, R. A. Buyanov, V. V. Molchanov, and L. M. Plyasova, “Morphology of carbon from methane on nickel-containing catalysts,” Catal Today, vol. 24, no. 3, 1995, doi: 10.1016/0920-5861(95)00040-M.
J. W. Snoeck, G. F. Froment, and M. Fowles, “Filamentous carbon formation and gasification: Thermodynamics, driving force, nucleation, and steady-state growth,” J Catal, vol. 169, no. 1, 1997, doi: 10.1006/jcat.1997.1634.